Targeting CDK9 for Anti-Cancer Therapeutics using PROTACs
- Rasraj Thoudam
- Aug 14, 2025
- 3 min read
Contents:
Abstract
PROTACs and its components
CDK9 – a promising target in cancer therapy
PROTAC synthesis using CuAAC click chemistry
CDK9 degradation efficiency by candidate PROTACs
CDK9 membrane permeability
The need for PROTACs
References
Abstract:
The development of PROTACs (Proteolysis Targeting Chimeras) has emerged as a promising strategy for targeting CDK9, a key player in cancer treatment. The application of click chemistry—specifically, Cu-mediated azide-alkyne cycloaddition (CuAAC)—for the in situ assembly of tumor-specific PROTACs has revolutionized synthesis, making it versatile and efficient. Beyond cancer, PROTACs are also being explored for non-cancer targets, including neurodegenerative, inflammatory, and autoimmune diseases, liver diseases, viral infections, cardiovascular diseases, metabolic disorders, and bacterial infections.
PROTACs and its components:
Proteolysis-targeting chimeras (PROTACs) are heterobifunctional small molecules designed to degrade a specific target protein (protein of interest/POI). They consist of two ligands:
A target protein warhead, which binds to the POI.
An E3 ligase ligand, which triggers POI degradation.
These two ligands are connected by a linker. A PROTAC brings the target protein into proximity with an E3 ligase, leading to ubiquitination and subsequent degradation via the ubiquitin-proteasome pathway.
CDK9 – a promising target in cancer therapy:
Cyclin-dependent kinase 9 (CDK9) is a member of the cyclin-dependent protein kinase (CDK) family that contributes to transcriptional elongation of multiple target genes. It is widely expressed and has been linked to cancers such as breast, prostate, and pancreatic cancer.
CDK9 phosphorylates RNA polymerase II, promoting transcriptional elongation of survival-critical genes, and ensures sustained expression of oncogenes such as MYC and MCL-1 in cancer cells. Inhibiting CDK9 can promote cell death in various blood and solid tumors.


PROTAC synthesis using CuAAC click chemistry:
Click chemistry refers to a set of highly efficient and selective reactions that form stable covalent bonds under mild conditions, commonly used in drug discovery and bioconjugation.
The CuAAC (Copper-Catalyzed Azide-Alkyne Cycloaddition) reaction forms 1,2,3-triazoles by reacting an azide with an alkyne in the presence of a copper catalyst. In PROTAC synthesis, CuAAC results in a triazole ring in the linker, improving compound stability and solubility.


CDK9 degradation efficiency by candidate PROTACs:
Western blotting is commonly used to confirm degradation of the target protein after PROTAC treatment. It detects the abundance of specific proteins semi-quantitatively and is typically employed for initial screening of candidate PROTAC efficiency.
The process involves lysing cells to extract total proteins, denaturing them with SDS, separating them by molecular weight through polyacrylamide gel electrophoresis, and transferring them to a membrane. The membrane is incubated with antibodies specific to the target protein, conjugated to detectable tags. Housekeeping proteins like β-actin or GAPDH serve as loading controls.

CDK9 membrane permeability:
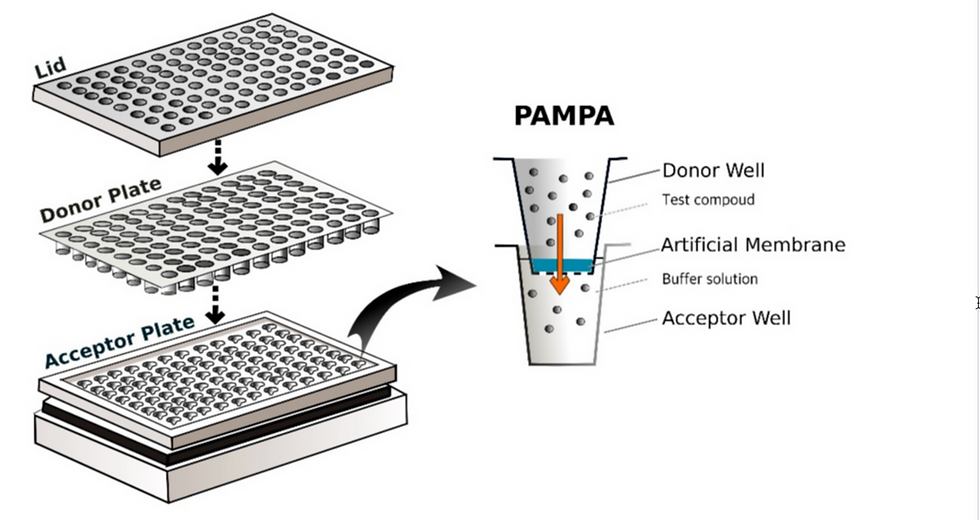
Membrane permeability assays confirm whether a PROTAC can enter cells. Two common methods are the PAMPA assay, which uses artificial membranes, and the Caco-2 assay, which assesses permeability across cell monolayers.
In PAMPA, the compound is loaded into a chamber separated by an artificial phospholipid-cholesterol membrane. After several hours, concentrations on both sides are measured by spectrophotometry or mass spectrometry. The effective permeability (Pe, cm/s), often expressed as –logPe, is calculated. Values above 6 indicate poor permeability; below 5 indicate good permeability, with 5–6 as intermediate.
The need for PROTACs:
Unlike traditional small-molecule inhibitors that rely on occupancy-driven inhibition, a single PROTAC can perform multiple degradation cycles—provided the target-binding moiety is non-covalent. This catalytic action enables effective degradation at sub-stoichiometric concentrations and doesn’t require high-affinity binding.
Additionally, small-molecule inhibitors must bind at specific sites to impair protein function, whereas PROTACs simply need to induce formation of a productive ternary complex between the target and an E3 ligase. This makes them especially suitable for degrading “undruggable” proteins lacking conventional drug-binding pockets, as long as suitable ligands can be developed.
References
Yang C, Tripathi R, Wang B. Click chemistry in the development of PROTACs. RSC Chem Biol. 2023.
Liu Z, Hu M, Yang Y, Du C, Zhou H, Liu C, Chen Y, Fan L, Ma H, Gong Y, Xie Y. An overview of PROTACs: a promising drug discovery paradigm. Mol Biomed. 2022 Dec.
Crummy EK, Caine EA, Mikheil D, Corona C, Riching KM, Hosfield C, Urh M. Monitoring PROTAC interactions in biochemical assays using Lumit immunoassays.
Pasieka A, Diamanti E, Uliassi E, Bolognesi ML. Click Chemistry and Targeted Degradation: A Winning Combination for Medicinal Chemists?. ChemMedChem. 2023.
Mandal R, Becker S, Strebhardt K. Targeting CDK9 for Anti-Cancer Therapeutics. Cancers (Basel). 2021 May.
Robb CM, Contreras JI, Kour S, Taylor MA, Abid M, Sonawane YA, Zahid M, Murry DJ, Natarajan A, Rana S. Chemically induced degradation of CDK9 by a proteolysis targeting chimera (PROTAC).



Comments